Overview
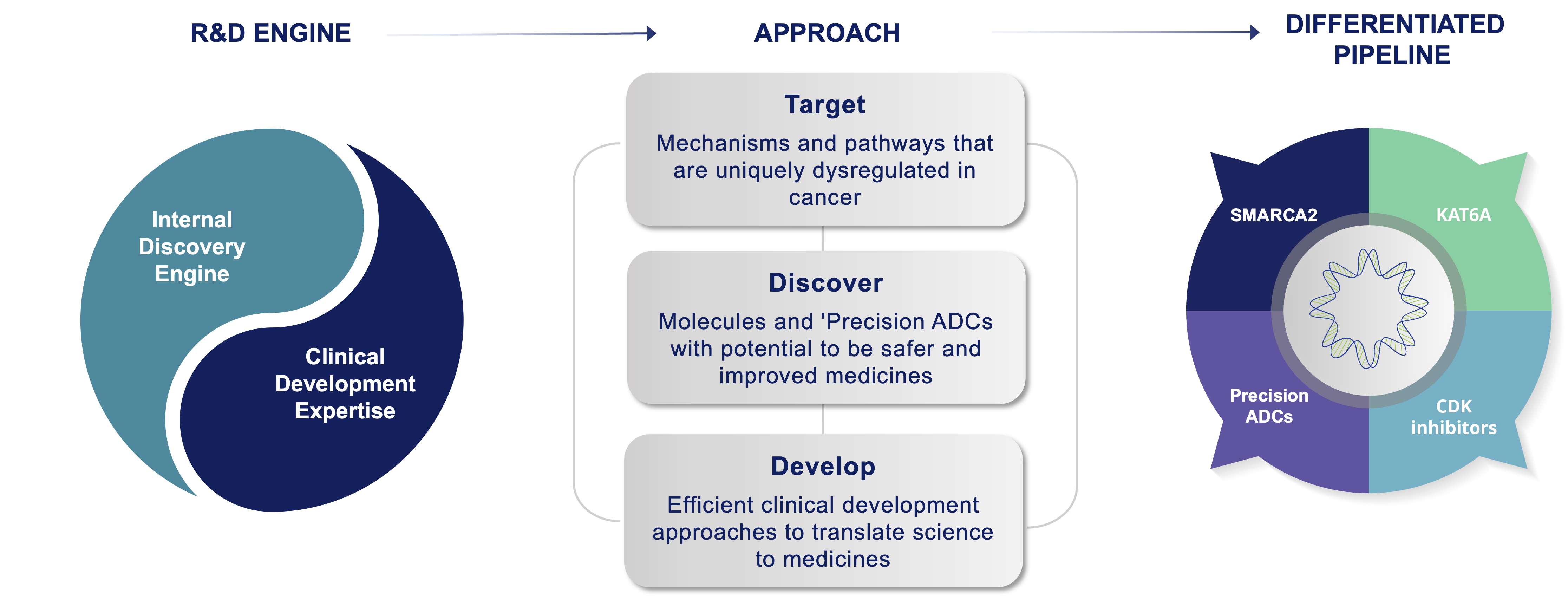
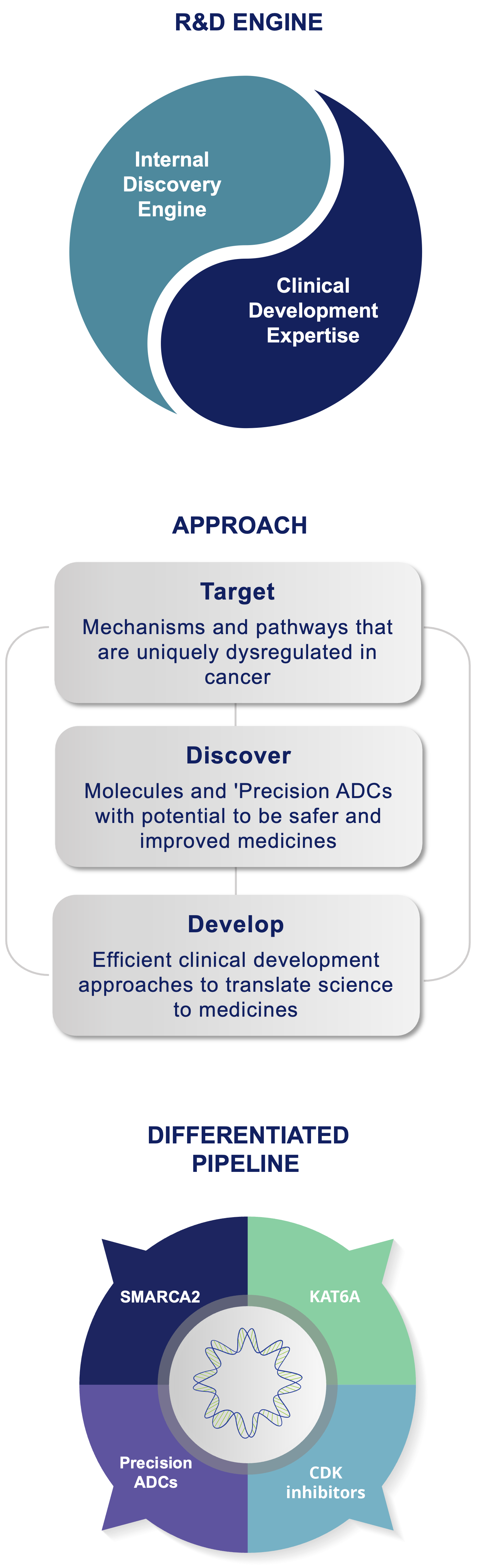
Prelude’s approach is target class and technology platform-agnostic, which means we do not limit our selection of programs to a defined target class, chemical modality, or a technology platform. Instead, Prelude applies its deep expertise in cancer biology and medicinal chemistry to identify targetable intervention points in cancer signaling pathways amenable to investigational small molecule-based therapies. Once an optimal target is identified, Prelude employs its internal discovery engine and extensive understanding of the current oncology treatment landscape to design and synthesize novel inhibitors or degraders with optimized properties. Prelude’s clinical development programs are focused on early proof of concept and efficient regulatory pathways that enable potentially transformative medicines to quickly reach patients with high unmet medical needs.
Prelude Discovery and Development Engine: Positioned to Succeed