SMARCA2
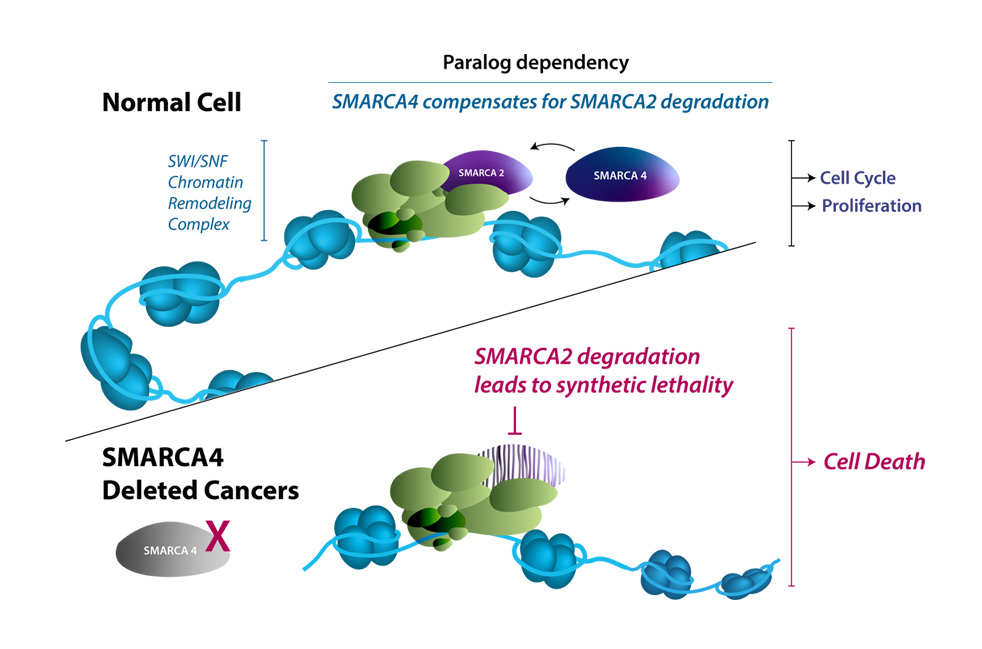
SMARCA2 and SMARCA4 (also known as BRM and BRG1, respectively) are involved in the regulation of gene expression through chromatin remodeling. SMARCA4 has been shown to be mutated in multiple cancers, including up to 10% of all non-small cell lung cancers (NSCLC). Because the activity of either SMARCA2 or SMARCA4 is required for chromatin remodeling to occur, SMARCA4-deficient cancer cells become highly dependent on SMARCA2 for their survival. Targeting SMARCA2 in SMARCA4-deficient cancers is believed to produce a strong synthetic lethality, resulting in SMARCA4-mutant tumor cell death while sparing normal cells that express SMARCA4 protein.
Structural similarities between SMARCA2 and SMARCA4 have historically posed challenges for selective SMARCA2 inhibition. Targeted protein degradation (TPD) is a relatively new approach that utilizes the endogenous protein degradation machinery, proteasome, to destroy oncogenic proteins and has been shown to provide highly selective degradation of SMARCA2 over SMARCA4. Using the TPD approach, Prelude is developing highly potent and selective SMARCA2 protein degraders designed to specifically inhibit SMARCA4-deficient tumors.
SMARCA4 & SMARCA2 Regulate Chromatin Accessibility & Gene Expression
PRT3789
PRT3789 is a potent, once-weekly IV degrader of SMARCA2 that demonstrates >1000-fold selectivity for SMARCA2 relative to SMARCA4 in cell-based assays. PRT3789 specifically inhibits SMARCA4-deficient human NSCLC cell lines in vitro and in vivo at well tolerated doses. PRT3789 is currently being evaluated in patients with SMARCA4-deficient solid tumors in a Phase I clinical trial, alone and in combination with docetaxel. PRT3789 represents the industry’s first clinical data on this new mechanism and will be presented at a major medical meeting in 2H 2024.
PRT7732
PRT7732 is a potent, orally administered degrader of SMARCA2 that demonstrates >3000-fold selectivity for SMARCA2 relative to SMARCA4 in cell-based assays. PRT7732 specifically inhibits SMARCA4-deficient human NSCLC cell lines in vitro and in vivo at well tolerated doses. PRT7732 is on track to begin Phase I clinical development in the 2H 2024.
